Electrolysis experiment method
If you’re searching for electrolysis experiment method images information related to the electrolysis experiment method keyword, you have visit the right site. Our site frequently gives you suggestions for viewing the maximum quality video and picture content, please kindly search and locate more enlightening video articles and images that match your interests.
Electrolysis Experiment Method. 2h o 2e h 2oh. Add two graphite rods as the electrodes and connect this to a power pack or battery. Can investigate if solution is an electrolyte by including a bulb in the circuit. If the bulb lights up the substance can conduct electricity and it is an electrolyte.
 Electrolysis Experimental Apparatus Download Scientific Diagram From researchgate.net
Electrolysis Experimental Apparatus Download Scientific Diagram From researchgate.net
2h o 4e o 4h. Often used to obtain elements that are too chemically reactive to be found free in nature. In the course of elec trol y sis the medi um of the elec trodes changes. 2h o 2e h 2oh. Can investigate if solution is an electrolyte by including a bulb in the circuit. Turn on power pack or battery and allow electrolysis to take place.
Turn on power pack or battery and allow electrolysis to take place.
Often used to obtain elements that are too chemically reactive to be found free in nature. 2h o 2e h 2oh. Add two graphite rods as the electrodes and connect this to a power pack or battery. 2h o 4e o 4h. In this experiment electrolysis will be used to separate water into hydrogen gas and oxygen gas. Turn on power pack or battery and allow electrolysis to take place.

Electrolysis is the process by which an electric current is passed through a substance to affect a chemical change. Can investigate if solution is an electrolyte by including a bulb in the circuit. Often used to obtain elements that are too chemically reactive to be found free in nature. Turn on power pack or battery and allow electrolysis to take place. Add two graphite rods as the electrodes and connect this to a power pack or battery.
 Source: youtube.com
Source: youtube.com
2h o 2e h 2oh. The chemical change occurs when the substance loses electrons oxidation or gains them reduction. In the course of elec trol y sis the medi um of the elec trodes changes. 2h o 2e h 2oh. Often used to obtain elements that are too chemically reactive to be found free in nature.

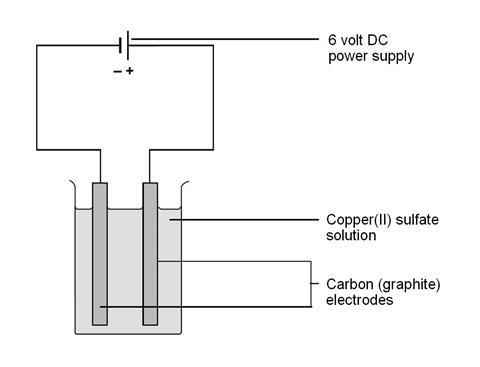
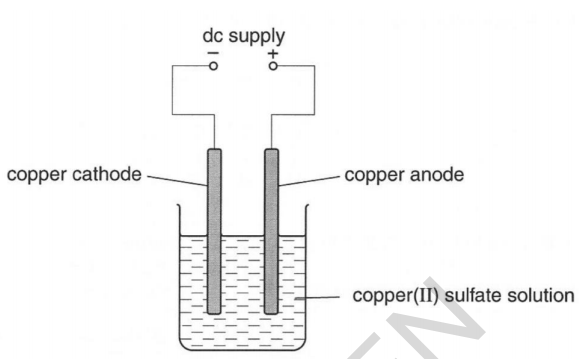
Turn on power pack or battery and allow electrolysis to take place. In this experiment electrolysis will be used to separate water into hydrogen gas and oxygen gas. Electrolysis is the process by which an electric current is passed through a substance to affect a chemical change. In the two experiments listed below the first reactive substance is water and the second one is a copper sulfate solution. Add two graphite rods as the electrodes and connect this to a power pack or battery.
 Source: study.com
Source: study.com
In this experiment electrolysis will be used to separate water into hydrogen gas and oxygen gas. If the bulb lights up the substance can conduct electricity and it is an electrolyte. Electrolysis is the process by which an electric current is passed through a substance to affect a chemical change. 2h o 4e o 4h. Turn on power pack or battery and allow electrolysis to take place.
 Source: edu.rsc.org
Source: edu.rsc.org
Turn on power pack or battery and allow electrolysis to take place. If the bulb lights up the substance can conduct electricity and it is an electrolyte. In the course of elec trol y sis the medi um of the elec trodes changes. In the two experiments listed below the first reactive substance is water and the second one is a copper sulfate solution. It be comes al ka line by the cath ode and the lit mus turns blue and by the an ode it be comes acidic and the lit mus turns red.
 Source: elevise.co.uk
Source: elevise.co.uk
The chemical change occurs when the substance loses electrons oxidation or gains them reduction. In the course of elec trol y sis the medi um of the elec trodes changes. Turn on power pack or battery and allow electrolysis to take place. In the two experiments listed below the first reactive substance is water and the second one is a copper sulfate solution. It be comes al ka line by the cath ode and the lit mus turns blue and by the an ode it be comes acidic and the lit mus turns red.
Source: en.wikipedia.org
Can investigate if solution is an electrolyte by including a bulb in the circuit. In this experiment electrolysis will be used to separate water into hydrogen gas and oxygen gas. Often used to obtain elements that are too chemically reactive to be found free in nature. It be comes al ka line by the cath ode and the lit mus turns blue and by the an ode it be comes acidic and the lit mus turns red. Can investigate if solution is an electrolyte by including a bulb in the circuit.
 Source: sciencedirect.com
Source: sciencedirect.com
Turn on power pack or battery and allow electrolysis to take place. Often used to obtain elements that are too chemically reactive to be found free in nature. Can investigate if solution is an electrolyte by including a bulb in the circuit. In the course of elec trol y sis the medi um of the elec trodes changes. Turn on power pack or battery and allow electrolysis to take place.
 Source: melscience.com
Source: melscience.com
2h o 2e h 2oh. 2h o 4e o 4h. In the two experiments listed below the first reactive substance is water and the second one is a copper sulfate solution. In this experiment electrolysis will be used to separate water into hydrogen gas and oxygen gas. 2h o 2e h 2oh.
 Source: scielo.br
Source: scielo.br
2h o 2e h 2oh. Add two graphite rods as the electrodes and connect this to a power pack or battery. Turn on power pack or battery and allow electrolysis to take place. If the bulb lights up the substance can conduct electricity and it is an electrolyte. 2h o 2e h 2oh.
 Source: www2.ucdsb.on.ca
Source: www2.ucdsb.on.ca
Can investigate if solution is an electrolyte by including a bulb in the circuit. 2h o 2e h 2oh. Often used to obtain elements that are too chemically reactive to be found free in nature. Can investigate if solution is an electrolyte by including a bulb in the circuit. Turn on power pack or battery and allow electrolysis to take place.

Often used to obtain elements that are too chemically reactive to be found free in nature. Add two graphite rods as the electrodes and connect this to a power pack or battery. The chemical change occurs when the substance loses electrons oxidation or gains them reduction. Often used to obtain elements that are too chemically reactive to be found free in nature. 2h o 2e h 2oh.
 Source: docbrown.info
Source: docbrown.info
If the bulb lights up the substance can conduct electricity and it is an electrolyte. 2h o 4e o 4h. In this experiment electrolysis will be used to separate water into hydrogen gas and oxygen gas. Turn on power pack or battery and allow electrolysis to take place. If the bulb lights up the substance can conduct electricity and it is an electrolyte.
 Source: docbrown.info
Source: docbrown.info
Can investigate if solution is an electrolyte by including a bulb in the circuit. Often used to obtain elements that are too chemically reactive to be found free in nature. 2h o 4e o 4h. If the bulb lights up the substance can conduct electricity and it is an electrolyte. The chemical change occurs when the substance loses electrons oxidation or gains them reduction.
 Source: researchgate.net
Source: researchgate.net
2h o 4e o 4h. Can investigate if solution is an electrolyte by including a bulb in the circuit. If the bulb lights up the substance can conduct electricity and it is an electrolyte. Electrolysis is the process by which an electric current is passed through a substance to affect a chemical change. In this experiment electrolysis will be used to separate water into hydrogen gas and oxygen gas.
If you find this site helpful, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title electrolysis experiment method by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.