Electroplating of copper experiment
If you’re looking for electroplating of copper experiment pictures information related to the electroplating of copper experiment keyword, you have pay a visit to the ideal blog. Our site frequently provides you with suggestions for seeking the maximum quality video and picture content, please kindly search and find more enlightening video articles and graphics that fit your interests.
Electroplating Of Copper Experiment. How the changing of current affects the electroplating of copper. When the experiment ends the electrodes are dried and the mass of each electrode weighed on the mini balance. The cathode gains mass the anode looses mass. The esthetic goal is to determine the suitability of several different commonly used coloring processes.
 An Easy Copper Electroplating Demo For Your Redox Unit Chemical Education Xchange From chemedx.org
An Easy Copper Electroplating Demo For Your Redox Unit Chemical Education Xchange From chemedx.org
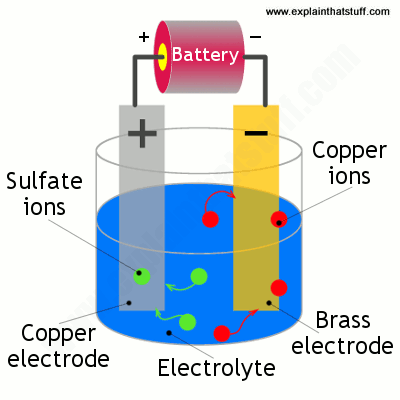
Copper electroplating requires electricty. Welcome to science at home in this experiment we are exploring copper electroplating by coating a nail with copper. Copper atoms on the anode are oxidized to copper ii ions. Your solution should be dark blue. Copper that is subsequently treated to produce a light green patina. When current is applied to the electrolysis cell copper ii ions in solution are reduced to copper atoms at the cathode.
Copper that is subsequently treated to produce a light green patina.
Welcome to science at home in this experiment we are exploring copper electroplating by coating a nail with copper. Welcome to science at home in this experiment we are exploring copper electroplating by coating a nail with copper. Copper atoms on the anode are oxidized to copper ii ions. When current is applied to the electrolysis cell copper ii ions in solution are reduced to copper atoms at the cathode. When the experiment ends the electrodes are dried and the mass of each electrode weighed on the mini balance. Electroplating experiment aim to find the amount copper gains or loses on the electrodes using different amounts of current each time during electrolysis.
 Source: yenka.com
Source: yenka.com
The cathode gains mass the anode looses mass. When the experiment ends the electrodes are dried and the mass of each electrode weighed on the mini balance. The cathode gains mass the anode looses mass. Welcome to science at home in this experiment we are exploring copper electroplating by coating a nail with copper. Your solution should be dark blue.
 Source: cikguwong.blogspot.com
Source: cikguwong.blogspot.com
Welcome to science at home in this experiment we are exploring copper electroplating by coating a nail with copper. The scientific goal of this experiment is to determine the efficiency of copper electroplating on nickel coated steel or brass. The esthetic goal is to determine the suitability of several different commonly used coloring processes. Electroplating is an energy intensive process. The cathode gains mass the anode looses mass.
 Source: explainthatstuff.com
Source: explainthatstuff.com
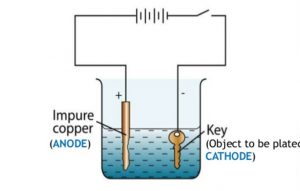
Use one alligator clip to attach the copper electrode to the positive terminal of the battery this is now the anode and the other to attach the key to the negative terminal now called the cathode. The scientific goal of this experiment is to determine the efficiency of copper electroplating on nickel coated steel or brass. When the experiment ends the electrodes are dried and the mass of each electrode weighed on the mini balance. Introduction electroplating is generally carried out in order to improve the. How the changing of current affects the electroplating of copper.
 Source: researchgate.net
Source: researchgate.net
When the experiment ends the electrodes are dried and the mass of each electrode weighed on the mini balance. Electroplating experiment aim to find the amount copper gains or loses on the electrodes using different amounts of current each time during electrolysis. Introduction electroplating is generally carried out in order to improve the. The scientific goal of this experiment is to determine the efficiency of copper electroplating on nickel coated steel or brass. Stir copper sulfate into some hot water in a beaker until no more will dissolve.
 Source: chemedx.org
Source: chemedx.org
When current is applied to the electrolysis cell copper ii ions in solution are reduced to copper atoms at the cathode. Welcome to science at home in this experiment we are exploring copper electroplating by coating a nail with copper. When current is applied to the electrolysis cell copper ii ions in solution are reduced to copper atoms at the cathode. The scientific goal of this experiment is to determine the efficiency of copper electroplating on nickel coated steel or brass. Introduction electroplating is generally carried out in order to improve the.
 Source: examfear.com
Source: examfear.com
How the changing of current affects the electroplating of copper. Copper atoms on the anode are oxidized to copper ii ions. Stir copper sulfate into some hot water in a beaker until no more will dissolve. The esthetic goal is to determine the suitability of several different commonly used coloring processes. Introduction electroplating is generally carried out in order to improve the.
 Source: revision.co.zw
Source: revision.co.zw
The scientific goal of this experiment is to determine the efficiency of copper electroplating on nickel coated steel or brass. When the experiment ends the electrodes are dried and the mass of each electrode weighed on the mini balance. Electroplating is an energy intensive process. Electroplating experiment aim to find the amount copper gains or loses on the electrodes using different amounts of current each time during electrolysis. Your solution should be dark blue.
 Source: chemdemos.uoregon.edu
Source: chemdemos.uoregon.edu
How the changing of current affects the electroplating of copper. Copper that is subsequently treated to produce a light green patina. Introduction electroplating is generally carried out in order to improve the. When the experiment ends the electrodes are dried and the mass of each electrode weighed on the mini balance. Copper electroplating requires electricty.
 Source: slideshare.net
Source: slideshare.net
The esthetic goal is to determine the suitability of several different commonly used coloring processes. Welcome to science at home in this experiment we are exploring copper electroplating by coating a nail with copper. Stir copper sulfate into some hot water in a beaker until no more will dissolve. The cathode gains mass the anode looses mass. When the experiment ends the electrodes are dried and the mass of each electrode weighed on the mini balance.
 Source: classnotes.org.in
Source: classnotes.org.in
Copper electroplating requires electricty. The scientific goal of this experiment is to determine the efficiency of copper electroplating on nickel coated steel or brass. Copper that is subsequently treated to produce a light green patina. Welcome to science at home in this experiment we are exploring copper electroplating by coating a nail with copper. Your solution should be dark blue.
 Source: docbrown.info
Source: docbrown.info
Introduction electroplating is generally carried out in order to improve the. Copper electroplating requires electricty. Introduction electroplating is generally carried out in order to improve the. Electroplating is an energy intensive process. Use one alligator clip to attach the copper electrode to the positive terminal of the battery this is now the anode and the other to attach the key to the negative terminal now called the cathode.
 Source: sites.google.com
Source: sites.google.com
Electroplating is an energy intensive process. Introduction electroplating is generally carried out in order to improve the. When current is applied to the electrolysis cell copper ii ions in solution are reduced to copper atoms at the cathode. Stir copper sulfate into some hot water in a beaker until no more will dissolve. Copper electroplating requires electricty.
 Source: m.youtube.com
Source: m.youtube.com
When the experiment ends the electrodes are dried and the mass of each electrode weighed on the mini balance. Copper atoms on the anode are oxidized to copper ii ions. The cathode gains mass the anode looses mass. Your solution should be dark blue. Electroplating is an energy intensive process.
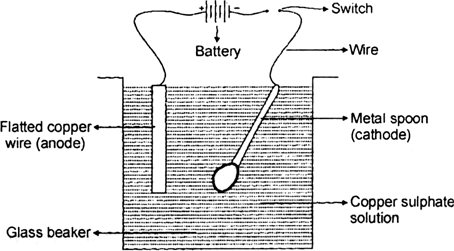 Source: zigya.com
Source: zigya.com
When current is applied to the electrolysis cell copper ii ions in solution are reduced to copper atoms at the cathode. How the changing of current affects the electroplating of copper. When the experiment ends the electrodes are dried and the mass of each electrode weighed on the mini balance. Welcome to science at home in this experiment we are exploring copper electroplating by coating a nail with copper. Stir copper sulfate into some hot water in a beaker until no more will dissolve.
 Source: docbrown.info
Source: docbrown.info
Copper electroplating requires electricty. Welcome to science at home in this experiment we are exploring copper electroplating by coating a nail with copper. How the changing of current affects the electroplating of copper. Copper that is subsequently treated to produce a light green patina. Use one alligator clip to attach the copper electrode to the positive terminal of the battery this is now the anode and the other to attach the key to the negative terminal now called the cathode.
If you find this site serviceableness, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title electroplating of copper experiment by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.