Sodium carbonate and water experiment
If you’re searching for sodium carbonate and water experiment pictures information related to the sodium carbonate and water experiment keyword, you have come to the ideal site. Our website frequently gives you hints for refferencing the maximum quality video and picture content, please kindly hunt and locate more enlightening video articles and images that match your interests.
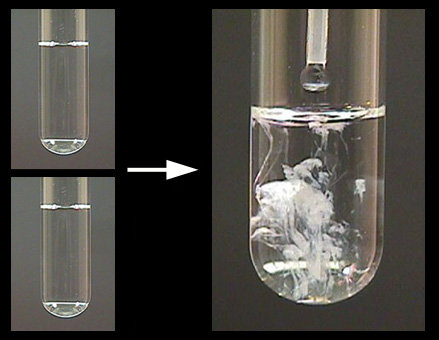
Sodium Carbonate And Water Experiment. When we did use sodium carbonate 0 5000g the water needed 4 0cm3 of soap solution to create a permanent lather and when we added 2 000g of sodium carbonate it only needed 3 0cm3 of soap solution to create a permanent lather. This shows that the more sodium carbonate you add the less soap is needed which means the water is softer. Sodium carbonate is an important commercial chemical. Sodium carbonate on a clock glass.
 Pdf Experimental No 08 Determination Of A Mixture Of Sodium Carbonate And Sodium Hydroxide By Using Double Indicator Method From researchgate.net
Pdf Experimental No 08 Determination Of A Mixture Of Sodium Carbonate And Sodium Hydroxide By Using Double Indicator Method From researchgate.net
Continue stirring the mixture with. To ensure that all the sodium carbonate is transferred use a wash bottle to rinse the clock glass with deionised water and add the rinsings to the beaker. It is also known as washing soda or soda ash. Hydrolysis is a reaction where a compound is split whilst incorporating a molecule of water. Sodium carbonate on a clock glass. We can get a lots of information from this science experiment.
Hydrolysis is a reaction where a compound is split whilst incorporating a molecule of water.
Uncertainty of equipment no. It is odorless and tasteless. In this experiment i predict that the sodium carbonate will soften the water and the more sodium carbonate added the softer the solution will get. In this video tutorial viewers learn how to do a sodium and water experiment. It is soluble in water. The latter two substances correspond of course to the titration of h 2co 3 to its flrst and second equivalence points.
 Source: studylib.net
Source: studylib.net
Sodium carbonate is an important commercial chemical. We can get a lots of information from this science experiment. Simply drop a piece of sodium into a cup of water. When exposed oxygen in the air an outer coding of sodium oxide will form. Continue stirring the mixture with.
 Source: researchgate.net
Source: researchgate.net
When dropped in water sodium reacts to form sodium hydroxide and hydrogen gas. To determine the mass of water in hydrated sodium carbonate salt and the value of x in na2co3 xh2o. When we did use sodium carbonate 0 5000g the water needed 4 0cm3 of soap solution to create a permanent lather and when we added 2 000g of sodium carbonate it only needed 3 0cm3 of soap solution to create a permanent lather. When exposed oxygen in the air an outer coding of sodium oxide will form. It occurs worldwide naturally and specifically in the minerals trona and nahcolite.
 Source: socratic.org
Source: socratic.org
2 1 carbon dioxide in aqueous solution. In this experiment i predict that the sodium carbonate will soften the water and the more sodium carbonate added the softer the solution will get. When dropped in water sodium reacts to form sodium hydroxide and hydrogen gas. In this section we will examine solutions of carbon dioxide sodium bicarbonate and sodium carbonate in pure water. Science experiment with water and eno explanation.
 Source: researchgate.net
Source: researchgate.net
Slowly transfer the sodium carbonate with stirring to about 50 cm 3 of deionised water in a clean 250 cm beaker. Sodium carbonate on a clock glass. To ensure that all the sodium carbonate is transferred use a wash bottle to rinse the clock glass with deionised water and add the rinsings to the beaker. Slowly transfer the sodium carbonate with stirring to about 50 cm 3 of deionised water in a clean 250 cm beaker. Sodium carbonate is a white crystalline powder or grayish white lumps.
 Source: youtube.com
Source: youtube.com
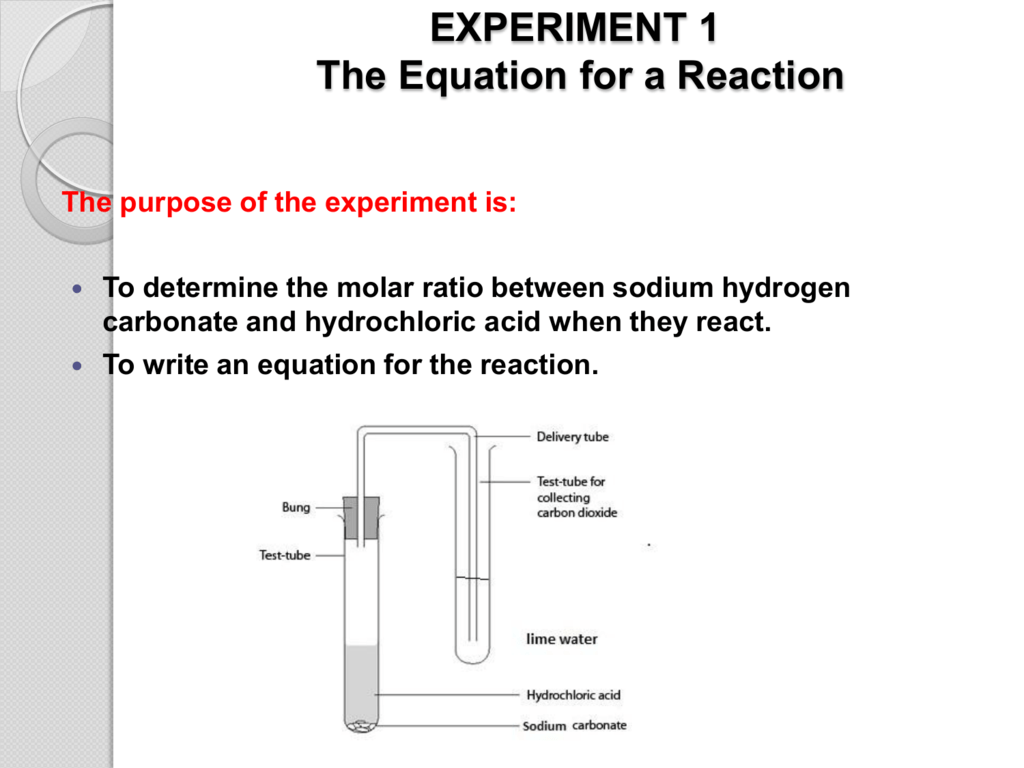
To determine the mass of water in hydrated sodium carbonate salt and the value of x in na2co3 xh2o. To determine the mass of water in hydrated sodium carbonate salt and the value of x in na2co3 xh2o. When dropped in water sodium reacts to form sodium hydroxide and hydrogen gas. Uncertainty of equipment no. It occurs worldwide naturally and specifically in the minerals trona and nahcolite.
 Source: studylib.net
Source: studylib.net
It is odorless and tasteless. Sodium is a silver metal that is very reactive. Slowly transfer the sodium carbonate with stirring to about 50 cm 3 of deionised water in a clean 250 cm beaker. It is classified as an acido basic reaction h exchange followed by a dehydration reaction. Eno is medicine drug that contains sodium carbonate and sodium bicarbonate that reacts with acid and release gas.
 Source: youtube.com
Source: youtube.com
The latter two substances correspond of course to the titration of h 2co 3 to its flrst and second equivalence points. Eno is medicine drug that contains sodium carbonate and sodium bicarbonate that reacts with acid and release gas. To determine the mass of water in hydrated sodium carbonate salt and the value of x in na2co3 xh2o. In this section we will examine solutions of carbon dioxide sodium bicarbonate and sodium carbonate in pure water. Slowly transfer the sodium carbonate with stirring to about 50 cm 3 of deionised water in a clean 250 cm beaker.

It is soluble in water. Sodium carbonate is a white crystalline powder or grayish white lumps. I predict that with no sodium carbonate to soften the water the water will need at least 6cm3 of soap solution to use up the calcium ions and show that the water has been softened. In this experiment i predict that the sodium carbonate will soften the water and the more sodium carbonate added the softer the solution will get. Sodium carbonate is an important commercial chemical.
 Source: youtube.com
Source: youtube.com
To ensure that all the sodium carbonate is transferred use a wash bottle to rinse the clock glass with deionised water and add the rinsings to the beaker. Sodium is a silver metal that is very reactive. When exposed oxygen in the air an outer coding of sodium oxide will form. To determine the mass of water in hydrated sodium carbonate salt and the value of x in na2co3 xh2o. The latter two substances correspond of course to the titration of h 2co 3 to its flrst and second equivalence points.
 Source: markedbyteachers.com
Source: markedbyteachers.com
It occurs worldwide naturally and specifically in the minerals trona and nahcolite. 2 1 carbon dioxide in aqueous solution. It occurs worldwide naturally and specifically in the minerals trona and nahcolite. The sodium will constant move around in the water. It is soluble in water.
 Source: youtube.com
Source: youtube.com
To determine the mass of water in hydrated sodium carbonate salt and the value of x in na2co3 xh2o. Simply drop a piece of sodium into a cup of water. Sodium is a silver metal that is very reactive. In this section we will examine solutions of carbon dioxide sodium bicarbonate and sodium carbonate in pure water. When exposed oxygen in the air an outer coding of sodium oxide will form.
 Source: youtube.com
Source: youtube.com
Water plus eno reaction. The latter two substances correspond of course to the titration of h 2co 3 to its flrst and second equivalence points. Sodium carbonate is an important commercial chemical. Apparatus uncertainty 1 electronic balance 0 01g 2 250 0cm3 volumetric flask 0 6cm3 3 50 00cm3 burette 0 05cm3 4 25 00cm3 pipette 0. To determine the mass of water in hydrated sodium carbonate salt and the value of x in na2co3 xh2o.
 Source: thoughtco.com
Source: thoughtco.com
When dropped in water sodium reacts to form sodium hydroxide and hydrogen gas. Josephine yeh ibdp 1 chemistry hl 25 02 14 title. Science experiment with water and eno explanation. Continue stirring the mixture with. In this video tutorial viewers learn how to do a sodium and water experiment.
 Source: studylib.net
Source: studylib.net
Sodium is a silver metal that is very reactive. Simply drop a piece of sodium into a cup of water. It is soluble in water. Slowly transfer the sodium carbonate with stirring to about 50 cm 3 of deionised water in a clean 250 cm beaker. Apparatus uncertainty 1 electronic balance 0 01g 2 250 0cm3 volumetric flask 0 6cm3 3 50 00cm3 burette 0 05cm3 4 25 00cm3 pipette 0.
 Source: researchgate.net
Source: researchgate.net
Hydrolysis is a reaction where a compound is split whilst incorporating a molecule of water. It is classified as an acido basic reaction h exchange followed by a dehydration reaction. Simply drop a piece of sodium into a cup of water. Uncertainty of equipment no. 2 1 carbon dioxide in aqueous solution.
If you find this site beneficial, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title sodium carbonate and water experiment by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






